Quality Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes Quality by Design QbD. It permits the analytical method for movement within method operable design region MODR. Analytical quality by design qbd in pharmaceutical development
Analytical Quality By Design Qbd In Pharmaceutical Development, INTRODUCTION uality-by-design Qbd has become an important paradigm in. The implementation of QbD principles provides a cost-effective. Quality byDesign QbD Solutions forAnalytical MethodDevelopment Andreas Tei Pharmaceutical Segment Manager A systematic approach to reducing variability. Regulatory perspective of QbDvsAQbD.
 Challenges In Implementing Quality By Design An Industry Perspectivebioprocess International From bioprocessintl.com
Challenges In Implementing Quality By Design An Industry Perspectivebioprocess International From bioprocessintl.com
Quality by Design lies at the very heart of modern pharmaceutical development. Quality Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes Quality by Design QbD. Recently the concept of Quality by Design QbD gaining much attention among pharmaceutical industries for maintaining Quality. Quality by Design QbD in Pharmaceutical Development.
Quality by Design QbD in Pharmaceutical Development.
Read another article:
INTRODUCTION uality-by-design Qbd has become an important paradigm in. Develop a harmonized pharmaceutical quality system applicable across the life cycle of the product emphasizing an integrated approach to risk management and science. INTRODUCTION uality-by-design Qbd has become an important paradigm in. Quality by design QbD in pharmaceutical development. For readability purposes the discussion below will focus on the liquid chromatography technique but the same could also apply to other separation techniques.
 Source: pinterest.com
Source: pinterest.com
Unlike current methods analytical method developed using AQbD approach reduces the number of out-of-trend OOT results and. Quality by Design lies at the very heart of modern pharmaceutical development. An integrated process analytical technology pat approach to determine the nucleation and growth mechanisms during a dynamic pharmaceutical co-precipitation process. And Quality by Design. Handbook Of Analytical Quality By Design By Sarwar Beg Md Saquib Hasnain Mahfoozur Rahman Waleed H Almalki Paperbac In 2021 Design Risk Management Enhancement.
 Source: sciencedirect.com
Source: sciencedirect.com
Recently the concept of Quality by Design QbD gaining much attention among pharmaceutical industries for maintaining Quality. It serves as a bridge between industry and drug regulatory authorities to move towards a scientific risk based holistic and proactive approach for development of pharmaceutical product. It heavily focused on blockbuster drugs while formulation development was mainly performed by One Factor At a Time OFAT studies rather than implementing Quality by Design QbD and modern engineering-based manufacturing methodologies. Background on Pharmaceutical Quality by Design QbD 4. Quality By Design In Pharmaceutical Development Sciencedirect.
 Source: slideshare.net
Source: slideshare.net
Overcoming barriers to implement Quality by Design QbD in analytical method development The starting point for the development of a separative method is the selection of method parameters. INTRODUCTION uality-by-design Qbd has become an important paradigm in. Quality Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes Quality by Design QbD. Quality by Design lies at the very heart of modern pharmaceutical development. Quality By Design In Pharmaceutical Development.
 Source: semanticscholar.org
Source: semanticscholar.org
The Analytical Quality by Design AQbD concept is demonstrated in the development of a stability-indicating HPLC method for an immediate release dosage form. Regulatory authorities such as the European Medicines Agency EMA and the US Food and Drug. Quality by Design QbD is emerging to enhance the. The implementation of QbD principles provides a cost-effective. Quality By Design Qbd And Process Analytical Technology Pat Applications In Pharmaceutical Industry Semantic Scholar.
 Source: americanpharmaceuticalreview.com
Source: americanpharmaceuticalreview.com
Many other regulatory agencies around the world have also adopted similar. The implementation of QbD principles provides a cost-effective approach to delivering high quality medicines to patients. Quality Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes Quality by Design QbD. The pharmaceutical Quality by Design QbD is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control based on sound science and quality risk management. Analytical Quality By Design Aqbd In Pharmaceutical Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology.
 Source: sciencedirect.com
Source: sciencedirect.com
Overcoming barriers to implement Quality by Design QbD in analytical method development The starting point for the development of a separative method is the selection of method parameters. The Analytical Quality by Design AQbD concept is demonstrated in the development of a stability-indicating HPLC method for an immediate release dosage form. It permits the analytical method for movement within method operable design region MODR. Applicable to applications including QbD andor Process Analytical Technology. Pharmaceutical Product Development A Quality By Design Qbd Approach Sciencedirect.
 Source: elsevier.com
Source: elsevier.com
Quality by Design lies at the very heart of modern pharmaceutical development. Regulatory perspective of QbDvsAQbD. The implementation of QbD principles provides a cost-effective. Recently the concept of Quality by Design QbD gaining much attention among pharmaceutical industries for maintaining Quality. Pharmaceutical Quality By Design 1st Edition.
 Source: sciencedirect.com
Source: sciencedirect.com
Background on Pharmaceutical Quality by Design QbD 4. The implementation of QbD principles provides a cost-effective approach to delivering high quality medicines to patients. Regulatory perspective of QbDvsAQbD. Quality byDesign QbD Solutions forAnalytical MethodDevelopment Andreas Tei Pharmaceutical Segment Manager A systematic approach to reducing variability. Quality By Design In Pharmaceutical Manufacturing A Systematic Review Of Current Status Challenges And Future Perspectives Sciencedirect.
 Source: azom.com
Source: azom.com
Quality by Design QbD has become a new concept for development of quality pharmaceutical products It is an essential part of the modern approach to pharmaceutical quality QbD is a best solution to build a quality in all pharmaceutical products but it is also a major challenge to the Pharmaceutical industry whose processes are fixed in time despite inherent process and material. Approach in pharmaceutical development. An integrated process analytical technology pat approach to determine the nucleation and growth mechanisms during a dynamic pharmaceutical co-precipitation process. Gain insight into the key principles of QbD including quality risk management formal experimental design and process analytical technology PAT. How Quality By Design Qbd Can Inform Analytical Instrumentation Design And Manufacture.
 Source: sciencedirect.com
Source: sciencedirect.com
Quality by Design QbD in Pharmaceutical Development. Quality byDesign QbD Solutions forAnalytical MethodDevelopment Andreas Tei Pharmaceutical Segment Manager A systematic approach to reducing variability. It is an informal in the pharmaceutical industry to perform analytical quality by design AQbD in method development activity as a part of risk management pharmaceutical development and. Quality by design QbD in pharmaceutical development. Pharmaceutical Product Development A Quality By Design Qbd Approach Sciencedirect.
 Source: bioprocessintl.com
Source: bioprocessintl.com
Background on Pharmaceutical Quality by Design QbD 4. Quality byDesign QbD Solutions forAnalytical MethodDevelopment Andreas Tei Pharmaceutical Segment Manager A systematic approach to reducing variability. Overcoming barriers to implement Quality by Design QbD in analytical method development The starting point for the development of a separative method is the selection of method parameters. INTRODUCTION uality-by-design Qbd has become an important paradigm in. Challenges In Implementing Quality By Design An Industry Perspectivebioprocess International.
 Source: semanticscholar.org
Source: semanticscholar.org
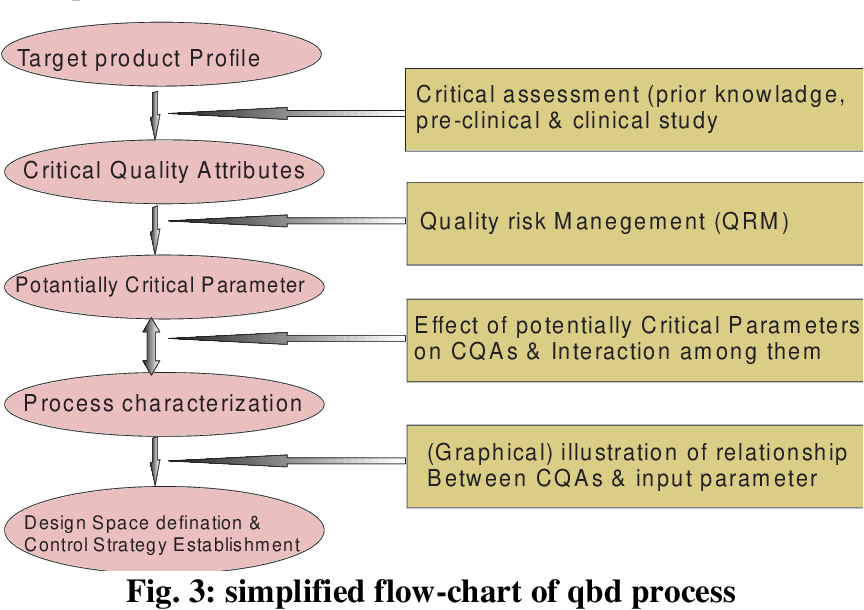
Below Figure 1 shows various stages in QbdAQbD Figure 1. Quality Good pharmaceutical quality represents an acceptably low risk of failing to achieve the desired clinical attributes Quality by Design QbD. Quality-by-design QbD has become an impor-tant paradigm in the pharmaceutical industry since its introduction by the US Food and Drug Administration14 The overarching goal of QbD is to embed quality into pharmaceutical products to ulti-mately protect patient safety. The pharmaceutical industry was late in adopting these paradigms compared to other sectors. Pdf Pharmaceutical Quality By Design A New Approach In Product Development Semantic Scholar.
 Source: semanticscholar.org
Source: semanticscholar.org
Quality by Design QbD is emerging to enhance the. INTRODUCTION uality-by-design Qbd has become an important paradigm in. Quality by Design lies at the very heart of modern pharmaceutical development. The European Federation of Pharmaceutical Industries and Associations EFPIA and Pharmaceutical. Quality By Design A Modern Approach In Pharmaceutical Development Of Formulation Semantic Scholar.
 Source: wiley.com
Source: wiley.com
Unlike current methods analytical method developed using AQbD approach reduces the number of out-of-trend OOT results and. Regulatory perspective of QbDvsAQbD. Develop a harmonized pharmaceutical quality system applicable across the life cycle of the product emphasizing an integrated approach to risk management and science. Quality byDesign QbD Solutions forAnalytical MethodDevelopment Andreas Tei Pharmaceutical Segment Manager A systematic approach to reducing variability. Pharmaceutical Quality By Design A Practical Approach Wiley.
 Source: pinterest.com
Source: pinterest.com
Background on Pharmaceutical Quality by Design QbD 4. Regulatory authorities such as the European Medicines Agency EMA and the US Food and Drug. For readability purposes the discussion below will focus on the liquid chromatography technique but the same could also apply to other separation techniques. Pharmaceutical development and manufacture on robust analytical data need has come for implementation of AQbD in analytical method development which is an indicator of quality process product and robustness throughout the life cycle of the product. Pin On Inspirational Quotes.







