Study Design Good experimental design enhances the power of the study Depends on. In this chapter we will discuss PD endpoint-based bioequivalence studies the general considerations for PD study design and validation and US Food and Drug Administration FDA recommendations. Bioequivalence study design fda
Bioequivalence Study Design Fda, This study design enabled us to determine the true intrasubject variability for the test and reference products independently and enabled us to apply the scaled-average-bioequivalence approach which offers more-flexible bioequivalence acceptance criteria for highly variable drug products according to the current EMACHMP Guidelines on the Investigation of. By Bob Pollock Aug 20 2021 Bioequivalence FDA Generics PSGs Regulatory Affairs. Example for crossover study design 7 Figure 5. Bioequivalence study provides bridging of the full clinical dataset held by Medsafe for the innovator medicine to support the efficacy and safety of generic medicines entering the New Zealand market.
 Brochure And Roll Up Design Getz Pharma On Behance Roll Up Design Pharma Brochure From in.pinterest.com
Brochure And Roll Up Design Getz Pharma On Behance Roll Up Design Pharma Brochure From in.pinterest.com
41 Design conduct and evaluation of bioequivalence studies The number of studies and study design depend on the physico-chemical characteristics of the substance its pharmacokinetic properties and proportionality in composition and should be justified. Show in their Journal of BE BA article El-Tahtawy A Harrison F Zirkelbach JF Jackson AJ 2011 Bioequivalence of Long Half-Life Drugs Informative Sampling Determination Parallel Designed Studies. 28 rows The selection of the method used to demonstrate bioequivalence depends upon the. This 42page guidance supersedes the December 2013 draft guidance of the.
8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug.
Read another article:
Example for crossover study design 7 Figure 5. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current. 90 CI of mean TR. 28 rows The selection of the method used to demonstrate bioequivalence depends upon the. 41 Design conduct and evaluation of bioequivalence studies The number of studies and study design depend on the physico-chemical characteristics of the substance its pharmacokinetic properties and proportionality in composition and should be justified.
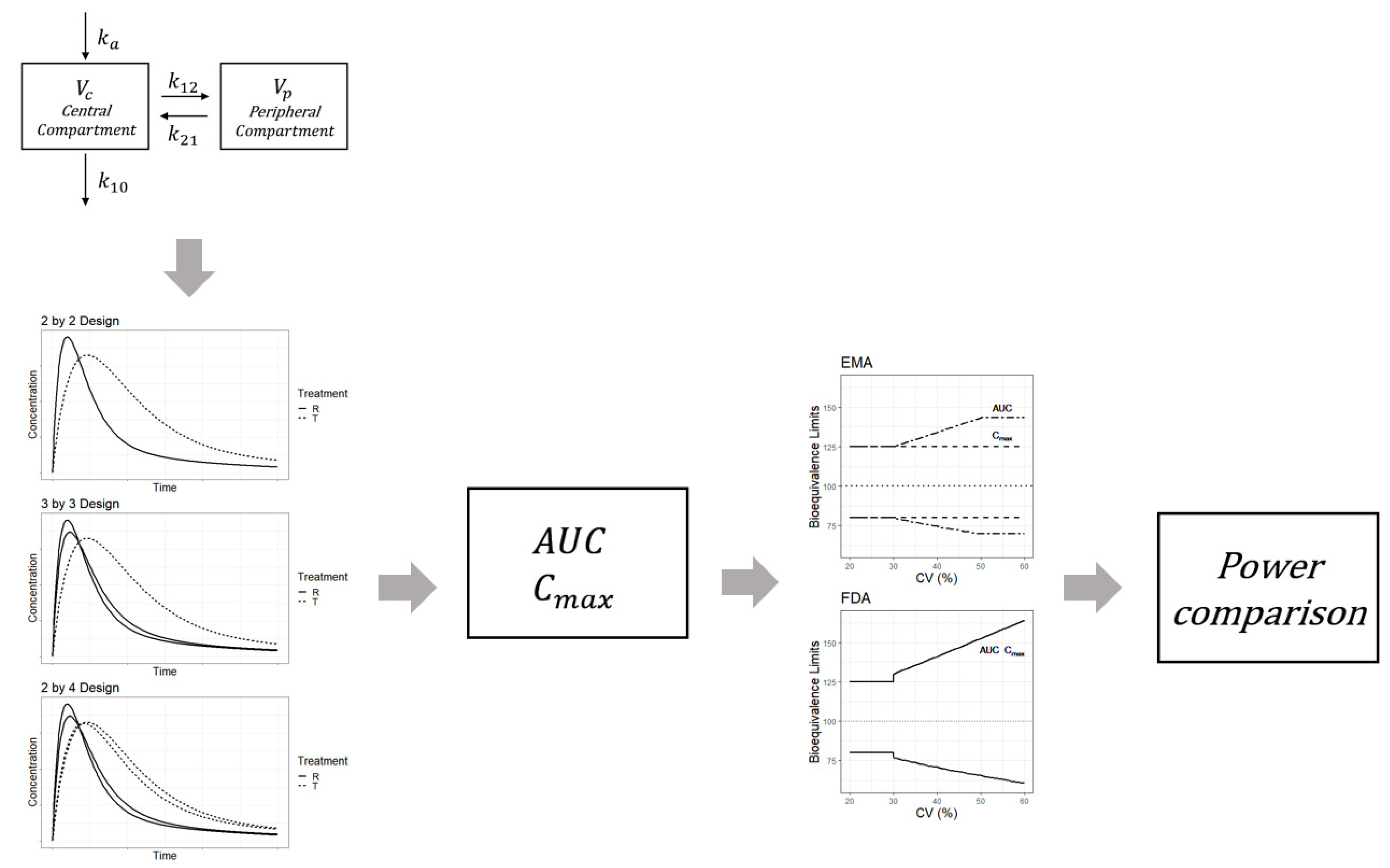 Source: mdpi.com
Source: mdpi.com
81 Clinical Study Design Study Design crossover parallel Fed Fasted Inclusion Exclusion Restriction Standardization of Study Condition Drug Administration Removal of Subject from Assessment Health Screening. The bioequivalence study uses anappropriate statistical assessment to determine whether. By Bob Pollock Aug 20 2021 Bioequivalence FDA Generics PSGs Regulatory Affairs. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current. Pharmaceuticals Free Full Text Model Based Approach For Designing An Efficient Bioequivalence Study For Highly Variable Drugs Html.
 Source: sciencedirect.com
Source: sciencedirect.com
It is related to the retention of reserve samples of the test article and reference standard used in an in vivo bioavailability BA and in vivo or in vitro bioequivalence BE tests. What is the justification for this. Bioequivalence BE studies are performed based on the requirements set forth in part 320 of section 21 of the Code of Federal Regulations CFR and guidance given by the US Food and Drug Administrations FDAs Center for Drug Evaluation and Research CDER 1. This guidance provides recommendations to study sponsors for the continuation or initiation of their bioequivalence BE studies during this COVID-19 public. Approaches To Supply Bioequivalent Oral Solid Pharmaceutical Formulations Through The Lifecycles Of Products Four Media Dissolution Monitoring Program In Japan Sciencedirect.
 Source: pinterest.com
Source: pinterest.com
Bioequivalence study provides bridging of the full clinical dataset held by Medsafe for the innovator medicine to support the efficacy and safety of generic medicines entering the New Zealand market. Bioequivalence study provides bridging of the full clinical dataset held by Medsafe for the innovator medicine to support the efficacy and safety of generic medicines entering the New Zealand market. Read together with Appendix IV. In this chapter we will discuss PD endpoint-based bioequivalence studies the general considerations for PD study design and validation and US Food and Drug Administration FDA recommendations. Untitled Lettering Letterhead Bid.
 Source: researchgate.net
Source: researchgate.net
By Bob Pollock Aug 20 2021 Bioequivalence FDA Generics PSGs Regulatory Affairs. This study design enabled us to determine the true intrasubject variability for the test and reference products independently and enabled us to apply the scaled-average-bioequivalence approach which offers more-flexible bioequivalence acceptance criteria for highly variable drug products according to the current EMACHMP Guidelines on the Investigation of. The bioequivalence study uses anappropriate statistical assessment to determine whether. Example for bioequivalence 6 Figure 4. A Visual Representation Of Some Possible Results Of The Statistical Download Scientific Diagram.
 Source: yumpu.com
Source: yumpu.com
Bioavailability and Bioequivalence Studies for Orally Administered Drug Products General Considerations This draft guidance represents the Food and Drug Administrations FDAs current. In this chapter we will discuss PD endpoint-based bioequivalence studies the general considerations for PD study design and validation and US Food and Drug Administration FDA recommendations. Today the FDA announced the issuance of a revised draft guidance titled Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry here. Planning BE studies as is the case in planning. Considerations For Planning And Designing A Bioequivalence Be.
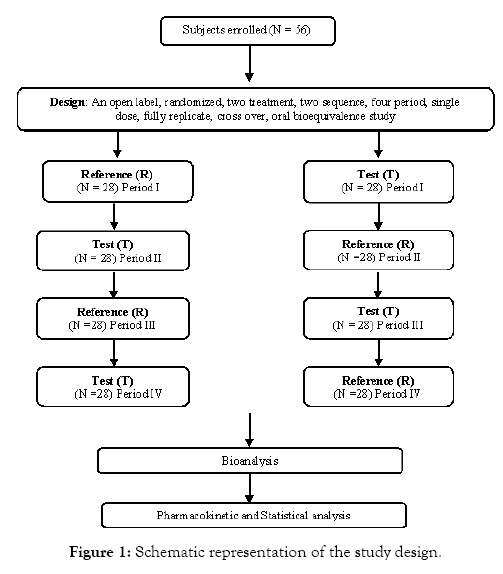 Source: longdom.org
Source: longdom.org
An applicant may request a waiver of in vivo bioequivalence testing for the 20 mg strength provided that it 1 submits. The US Food and Drug Administration FDA has released new guidance on the agencys compliance policy regarding samples used in bioavailability and bioequivalence studies. Example for bioequivalence 6 Figure 4. The FDA received a few comments related to truncation. A Full Replicate In Vivo Bioequivalence Study Of Two Idelalisib 150 Mg Tablets In Fasted Healthy Adult Human Subjects.
 Source: pinterest.com
Source: pinterest.com
8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. In this chapter we will discuss PD endpoint-based bioequivalence studies the general considerations for PD study design and validation and US Food and Drug Administration FDA recommendations. According to the current FDA guidance in vivo bioequivalence studies should be conducted in individuals 18 years or older who are representative of the general population taking into account for age sex and race. The bioequivalence study uses anappropriate statistical assessment to determine whether. Avoiding Risky Business Developing Effective Rems Plans Http Whybenchmarking Com 2013 07 05 Avoiding Risky Business Risky Business How To Plan Development.
 Source: slideshare.net
Source: slideshare.net
The Reference Listed Drug. According to the current FDA guidance in vivo bioequivalence studies should be conducted in individuals 18 years or older who are representative of the general population taking into account for age sex and race. What is the justification for this. 41 Design conduct and evaluation of bioequivalence studies The number of studies and study design depend on the physico-chemical characteristics of the substance its pharmacokinetic properties and proportionality in composition and should be justified. Bioequivalence Studies.
 Source: yumpu.com
Source: yumpu.com
Comparison of product-specific recommendations of EMA and US-FDA 53. The FDA received a few comments related to truncation. If the product is intended for use in both sexes inclusion of similar proportions of males and females should be intended. Example for crossover study design 7 Figure 5. Statistical Evaluation Of Bioequivalence Studies Bebac.
 Source: sciencedirect.com
Source: sciencedirect.com
FDA Issues Guidance on Bioequivalence Studies. Example for crossover study design 7 Figure 5. Conclusion of bioequivalence studies Study design appropri ate and study conduct satisfactory No critical deficiencies or abnormalities methods or statistical analysis Bioequivalence established. 8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. The Impact Of New Partial Auc Parameters For Evaluating The Bioequivalence Of Prolonged Release Formulations Sciencedirect.
 Source: slideshare.net
Source: slideshare.net
90 CI of mean TR. Comparison of product-specific recommendations of EMA and US-FDA 53. Bioequivalence pharmacokinetics biowaiver BCS-based biowaiver in vitro dissolution generics. Bioequivalence BE studies are performed based on the requirements set forth in part 320 of section 21 of the Code of Federal Regulations CFR and guidance given by the US Food and Drug Administrations FDAs Center for Drug Evaluation and Research CDER 1. Bioequivalence Studies A Statistical Approach Through R.
 Source: researchgate.net
Source: researchgate.net
Exemplary product-specific US-FDA recommendations 48 Table 2. FDA Issues Guidance on Bioequivalence Studies. 90 CI of mean TR. 28 rows The selection of the method used to demonstrate bioequivalence depends upon the. Fda Advisory Committee Discussion And Guidance On Recent Bioequivalence Download Table.
 Source: semanticscholar.org
Source: semanticscholar.org
All in all the bioequivalence studies should be designed to provide an objective means of critically assessing the possibility of alternative use of two drug products. According to the current FDA guidance in vivo bioequivalence studies should be conducted in individuals 18 years or older who are representative of the general population taking into account for age sex and race. 90 CI of mean TR. Exemplary product-specific US-FDA recommendations 48 Table 2. Pdf The Basic Regulatory Considerations And Prospects For Conducting Bioavailability Bioequivalence Ba Be Studies An Overview Semantic Scholar.
 Source: in.pinterest.com
Source: in.pinterest.com
In this chapter we will discuss PD endpoint-based bioequivalence studies the general considerations for PD study design and validation and US Food and Drug Administration FDA recommendations. Microsoft Word - Bioequivalence Study Reporting Format. In this chapter we will discuss PD endpoint-based bioequivalence studies the general considerations for PD study design and validation and US Food and Drug Administration FDA recommendations. Study Design Good experimental design enhances the power of the study Depends on. Brochure And Roll Up Design Getz Pharma On Behance Roll Up Design Pharma Brochure.
 Source: researchgate.net
Source: researchgate.net
The FDA received a few comments related to truncation. 8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. The Reference Listed Drug. Planning BE studies as is the case in planning. Percent Of Studies Passing Bioequivalence Be Power Curves Average Download Scientific Diagram.







