6 133 Pharmacokinetic characteristics. Microsoft PowerPoint - Bioequivalence Studies pp_ Nov 5_09ppt Author. Bioequivalence study design ppt.
Bioequivalence Study Design Ppt, Approval from Institutional Review Board IRB of the testing unit. Among various approaches to address the bioequivalence issue for highly variable drugs reference-scaled average BE approach has been suggestedThis approach requires less subjects in the study but with replicated treatment design such as three-period reference- replicated crossover design with sequences of TRR RTR RRT or four-period design with sequences of TRTR and RTRT. Activate the project by clicking its name in the Object Browser left side panel. In Vitro Bioequivalence BE Pathways Marilyn Martinez PhD Senior Biomedical Research Scientist Office of New Animal Drug Evaluation Center for Veterinary Medicine FDA.
 Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download From slideplayer.com
Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download From slideplayer.com
It includes randomized two-period two-sequence single dose cross-over design parallel design and replicate designs. 90 CI of mean TR. On-screen Show 43 Other titles. Bioequivalence studies are a surrogate marker for clinical effectiveness and safety data as it would not normally be practical to repeat clinical studies for generic products.
DESIGN OF BIOEQUIVALENCE STUDIES The test and reference drug formulations must contain.
Read another article:
Criterion for choosing an appropriate design is whether or not the selected design can identify estimate and isolate the intersubject variability. 6 133 Pharmacokinetic characteristics. If there are 20 subjects number the from 1 to 20. Open Access International Journal of. And then repeat for all other treatments.
 Source: slideserve.com
Source: slideserve.com
Scientific question and objectives to be answered 2. Avoids the ethical requirements of administering drugs to the healthy subjects. Choose Insert NCA and Toolbox Bioequivalence which will open a. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. Ppt Seminar On Crossover Design Powerpoint Presentation Free Download Id 3801391.
 Source: slideserve.com
Source: slideserve.com
DESIGN OF BIOEQUIVALENCE STUDIES The test and reference drug formulations must contain. Pk and Pd of drug substance 5. Bioequivalence studies are a surrogate marker for clinical effectiveness and safety data as it would not normally be practical to repeat clinical studies for generic products. Randomly select non repeating numbers among these labels for the first treatment. Ppt Bioequivalence General Considerations Powerpoint Presentation Free Download Id 1702423.
 Source: slideserve.com
Source: slideserve.com
A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. Bioequivalence studies are a surrogate marker for clinical effectiveness and safety data as it would not normally be practical to repeat clinical studies for generic products. Microsoft PowerPoint - Bioequivalence Studies pp_ Nov 5_09ppt Author. Pharmaceutically equivalent drug in the same dose strength in similar dosage forms eg immediate release or controlled release and given by the same route of administration. Ppt Seminar On Crossover Design Powerpoint Presentation Free Download Id 3801391.
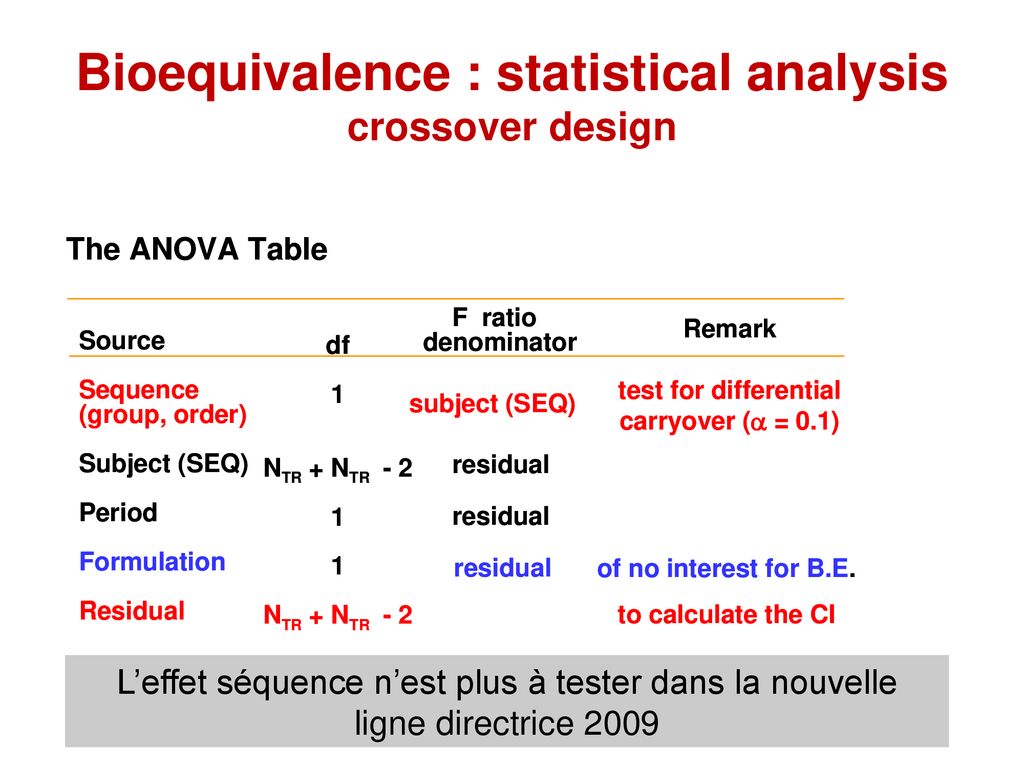 Source: slideplayer.fr
Source: slideplayer.fr
5 132 Single- vs. Among various approaches to address the bioequivalence issue for highly variable drugs reference-scaled average BE approach has been suggestedThis approach requires less subjects in the study but with replicated treatment design such as three-period reference- replicated crossover design with sequences of TRR RTR RRT or four-period design with sequences of TRTR and RTRT. Make a new Worksheet with the right headers for your bioequivalence data. All treatments are randomly allocated among all experimental subjects. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.
 Source: slideplayer.com
Source: slideplayer.com
Absolute and Relative bioavailabilty are discussed. 13 Design and conduct of bioequivalence studies. Bioequivalence studies are a surrogate marker for clinical effectiveness and safety data as it would not normally be practical to repeat clinical studies for generic products. On-screen Show 43 Other titles. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.
 Source: slideplayer.fr
Source: slideplayer.fr
This document specifies the requirements for the design conduct and evaluation of bioequivalence studies for immediate release dosage forms with systemic action. Pharmacokinetics and Pharmacodynamics of the study designs make an important role. Activate the project by clicking its name in the Object Browser left side panel. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.
 Source: slideshare.net
Source: slideshare.net
Bioequivalence studies comparing the product applied for with non-EU reference products should not be submitted and do not need to be included in the list of studies. Make a new Worksheet with the right headers for your bioequivalence data. Objective The basic design for bioequivalence study is determined by. Pk and Pd of drug substance 5. Bioequivalence.
 Source: slideplayer.fr
Source: slideplayer.fr
Absolute and Relative bioavailabilty are discussed. If there are 20 subjects number the from 1 to 20. Approval from Institutional Review Board IRB of the testing unit. Bioavailability and Boequivalenceppt - Free download as Powerpoint Presentation ppt PDF File pdf Text File txt or view presentation slides online. A Bioequivalence Considerations Techniques Et Scientifiques Ppt Telecharger.
 Source: slideplayer.com
Source: slideplayer.com
Drug absorption pattern in disease states can be evaluated. 90 CI of mean TR. Avoids the ethical requirements of administering drugs to the healthy subjects. Nature of reference material and dosage form to be tested 3. Bioequivalence Of Locally Acting Gi Drugs Ppt Video Online Download.
 Source: slideshare.net
Source: slideshare.net
On-screen Show 43 Other titles. Choose Insert NCA and Toolbox Bioequivalence which will open a. In Vitro Bioequivalence BE Pathways Marilyn Martinez PhD Senior Biomedical Research Scientist Office of New Animal Drug Evaluation Center for Veterinary Medicine FDA. Reflects better therapeutic efficacy of a drug. Bioequivalence.
 Source: slideserve.com
Source: slideserve.com
13 Design and conduct of bioequivalence studies. Bioequivalence studies comparing the product applied for with non-EU reference products should not be submitted and do not need to be included in the list of studies. WinnerLawrence Herman Created Date. In Vitro Bioequivalence BE Pathways Marilyn Martinez PhD Senior Biomedical Research Scientist Office of New Animal Drug Evaluation Center for Veterinary Medicine FDA. Ppt Anda Bioequivalence Studies That Fail To Meet Fda S Current Bioequivalence Criteria Powerpoint Presentation Id 325536.
 Source: slideplayer.com
Source: slideplayer.com
A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. 13 Design and conduct of bioequivalence studies. Avoids the ethical requirements of administering drugs to the healthy subjects. Scientific question and objectives to be answered 2. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.
 Source: slideshare.net
Source: slideshare.net
Approval from Institutional Review Board IRB of the testing unit. Randomly select non repeating numbers among these labels for the first treatment. International Journal of BioAnalytical Methods BioEquivalence Studies ISSN 2470-4490 - International Journal of BioAnalytical Methods BioEquivalence Studies IJBMBS ISSN 2470-4490 is a peer-reviewed open access journal wherein publication related to high-impact research articles contributes on the fundamental and applied topics of analytical and bioanalytical science including chromatography. Approval from Institutional Review Board IRB of the testing unit. Bioequivalence.
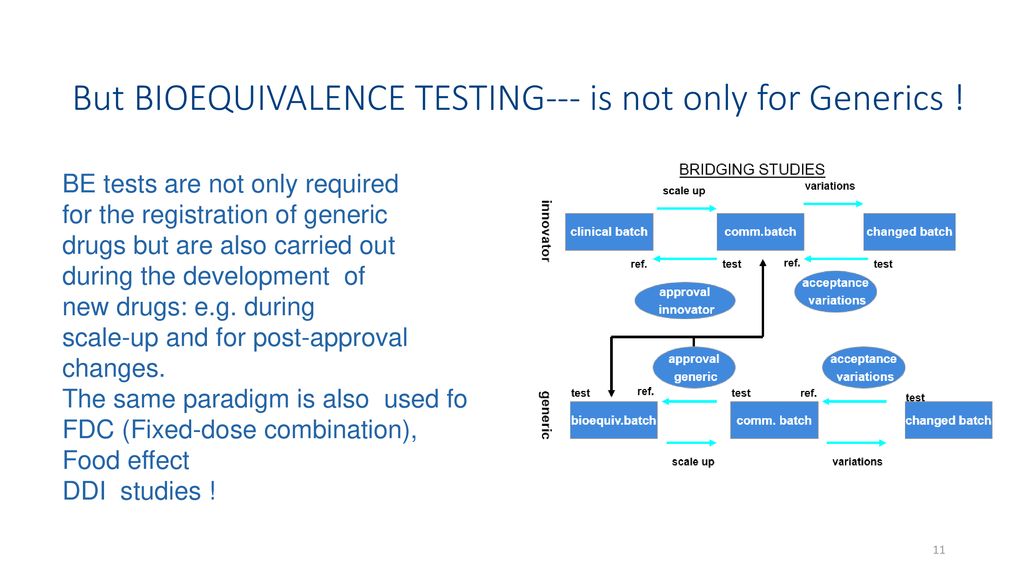 Source: slideplayer.com
Source: slideplayer.com
All treatments are randomly allocated among all experimental subjects. Avoids the ethical requirements of administering drugs to the healthy subjects. Approval from Institutional Review Board IRB of the testing unit. Bioequivalence Study Reporting Format 1. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.
 Source: slideplayer.com
Source: slideplayer.com
Presents the recent developments in methodology including population and individual bioequivalence. The basic assumption The basic assumption underlying the bioequivalence concept is that similar plasma time courses lead to essentially the same effects pharmacological toxic therapeutic Classical objections Plasma concentration is not biophase concentration there is no univocal relationships between. Open Access International Journal of. 5 132 Single- vs. Bioequivalence Trials Design Evaluation Regulatory Requirements Ppt Download.







